Graphite Mines
Plumbago - Black Lead



Graphite is a mineral consisting of nearly pure carbon C. Similar to coal but with much less impurities in form of ashes, water and sulfur. Coal is normally formed by moores growing continually by a continuous subsidence of the surface. So the trees and other plants do not decompose, but form huge layers of peat. Later this peat is pressed by sediments on top, also it becomes warmer and warmer because of heat of the earth. The longer this process takes and the higher the pressure and temperature are, the purer the carbon becomes. Brown coal or lignite is a coal of the lowest quality, pit coal or hard coal is a high quality coal, but graphite is nearly pure carbon. With even higher pressures and temperatures, diamonds would be formed.
The temperatures and pressures necessary to form graphite are high enough to also alterate the surrounding rocks. So graphite is typically found in coal beds, between layers of metamorphic limestones, marbles and carbon rich shales. The mineral graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. This means, graphite crystallizes in the hexagonal system.
Graphite burns, so it could be used as a fuel. Sometimes it is used to produce very hot fires in industrial processes. But normally the use of graphite is much broader, and it is rather rare and expensive (compared to coal). The most common use is also the explanation for the name: it is derived from the Greek word graphein, to write. Graphite is used to produce pencils by putting a thin bar of graphite into a wooden tube. It was named by the German chemist and mineralogist A. G. Werner in 1789. The name was derived from the ancient Greek γράφειν (graphein), which means to write, in reference to pencils. The German name Bleistift (pencil) derives from the fact that a large deposit of pure graphite was discovered in England, but they mistook it for a form of the lead mineral galena and called it plumbago. So pencils were named Blei(lead)stift, which stuck to this day, even though Carl Wilhelm Scheele proved as early as 1779 that graphite is pure carbon.
Graphite has been used in Europe since prehistoric times. In the Mesolithic period, it was used as a colouring agent and placed in graves with the dead. In the Neolithic period, it was used for ceramics, and in the Early Bronze Age, it had a variety of uses. In the Iron Age, graphite was used to make vessels more fire-resistant. Its modern uses include writing instruments and lubricants, paints and steel production. It is also used in electronics, nuclear reactors and batteries.
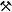 Grafitový důl Český Krumlov
Grafitový důl Český Krumlov Search DuckDuckGo for "Graphite Mine"
Search DuckDuckGo for "Graphite Mine" Graphite - Wikipedia (visited: 27-NOV-2025)
Graphite - Wikipedia (visited: 27-NOV-2025) Graphite at webmineral.com
Graphite at webmineral.com
 Index
Index Topics
Topics Hierarchical
Hierarchical Countries
Countries Maps
Maps